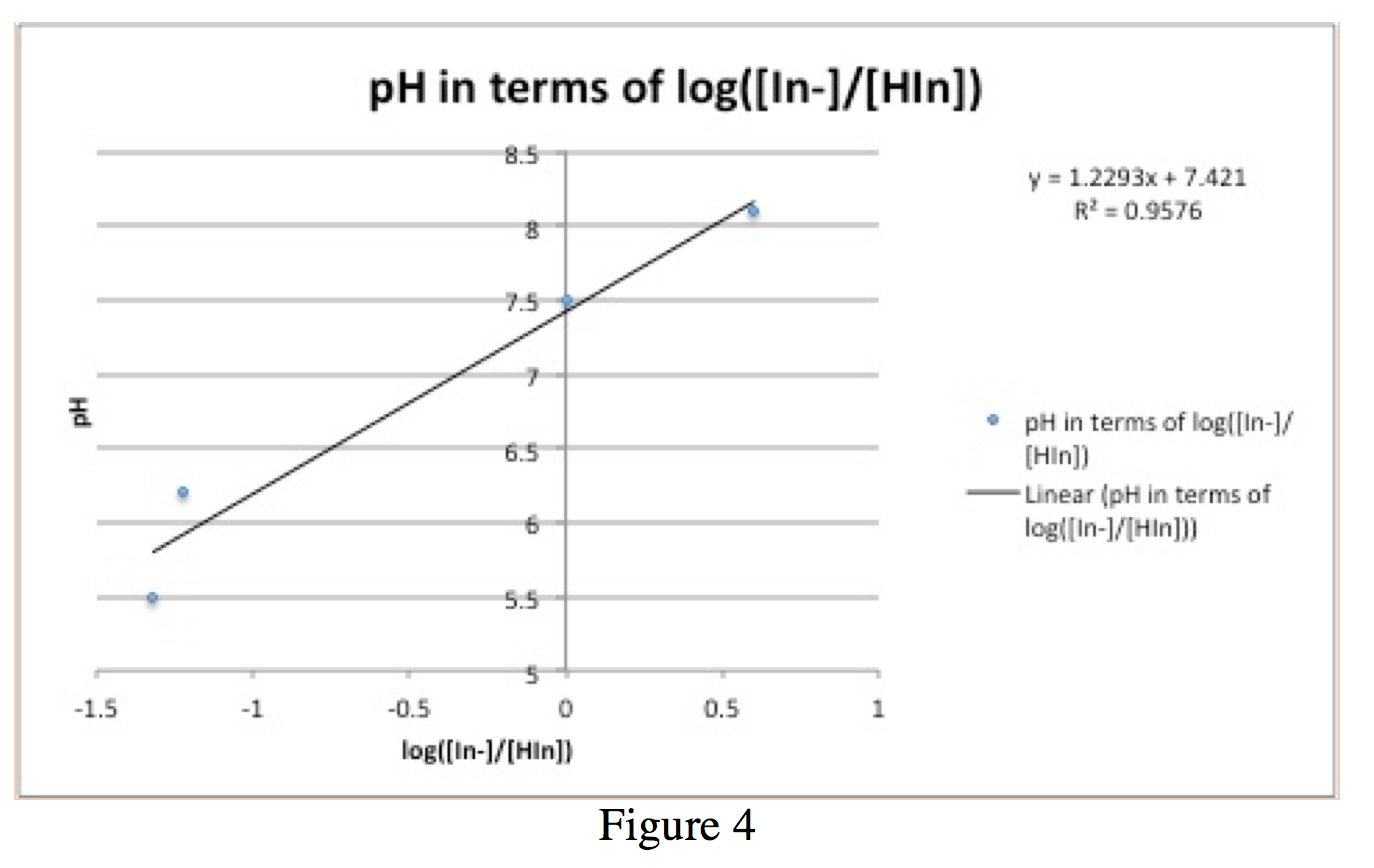
10 mM Tris pH 80 as the buffer. II - measurements which are made at a wavelength λ2 at which In-absorbs radiation.

Pdf Spectrophotometric Determination Of The Pka Isosbestic Point And Equation Of Absorbance Vs Ph For A Universal Ph Indicator Semantic Scholar
Bromothymol Blue Spectrophotometry Report Experiment
Uv Vis Spectroscopy Practice Questions Master Organic Chemistry
The absorbance and extinction ε are sometimes defined in terms of the natural logarithm instead of the base-10 logarithm.

Relationship between ph and absorbance pdf. The relationship between molar extinction coefficient ε. ἄνθος anthos flower and κυάνεος κυανοῦς kyaneoskyanous dark blue are water-soluble vacuolar pigments that depending on their pH may appear red purple blue or blackIn 1835 the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time. Molecules with an absorbance fluorescence excitation andor fluorescence emission spectrum sensitive to pH may be convenient probes for measuring pH i.
I- measurements which are made at a wavelength λ1 at which HIn absorbs radiation. For most applications absorbance values are used since the relationship between absorbance and both concentration and path length normally is linear. If a reaction changes the absorptivity of a sample this absorbance can be correlated to the rate of a reaction.
Measure the absorbance of the mixture in the cuvette every 20 seconds until the absorbance drops to 001. Germination rates were significantly higher after 24 h in running water than in controls Fig. If one exposes a solution of a pH-sensitive dye to light then dye molecules may absorb some of the light as electrons make the transition to a higher-energy state.
A graph of absorbance vs concentration is called a Beers Law curve in honor of the chemist who first discovered the relationship between absorbance and concentration. Further dilutions were then made using 10 mM Tris pH 80 as the diluent and 200 ml aliquots of each dilution were dispensed into microplate wells in replicates of 4. To correct for this prepare one or more control wells without cells and subtract the average absorbance of the control wells from that of the other wells.
The dilution factor is the inverse of the concentration factor. In this section we will learn how to construct titration curves for several important types of acid-base titrations. To understand the relationship between end points and equivalence points we must know how the pH changes during a titration.
This relationship is known as Beers law and is expressed mathematically as A. 20 one can observe an example of the UVvis spectral runs obtained for different sizes of silver nanoparticles. Background absorbance depends on the culture medium pH incubation time and length of exposure to light.
All clearly explained in. I have established pNP standard curve by taking pNP dilutions 0 to 1 mM 1 µmolmL in 20mM tris pH 6 optimum pH of my enzyme too. Relationship has been exploited for the spectrophotometric determination of protein concentrations and it is defined by the BeerLambert law or Beers law.
Solution created by the pH adjustment of an aqueous solution of 20 mM sodium dihydrogen phosphate. The absorbance of a solution depends on the quantity of light absorbing species in the solution and the length of the solution the light passes through. Rearranging equation 4 results in the most common form of.
Therefore the first step will be to determine the relationship between Absorbance of light at 405 nm and the concentration of p-nitrophenolate that is you will determine E for this compound at 405nm using a standard curve as in experiment 1. The BeerLambert Law is useful for characterizing many compounds but does not hold as a universal relationship for the concentration and absorption of all substances. For example Ag-NPs exhibit a specific absorbance peak between 400 and 450 nm Au-NPs between 500 and 550 nm and ZnO-NPs from 300 nm to 400 nm 3.
A spectrophotometer sends beams of light through a sample and with proper calibration measures the light absorbance of the sample. I need to use Megazyme pNP-oligosaccharides in couple of days. I Using the calibration curve convert the absorbance values obtained into concentrations of MnO 4-.
Molar and percent extinction coefficient ε. Full PDF Package Download Full PDF Package. The relationship between concentration and absorbance is not linear in BCAELISA assays.
Aεlc where l is the path length or the distance the light travels through a sample usually expressed in cm and ε. The pH at the end point however may or may not correspond to the pH at the equivalence point. The Coomassie brilliant blue protein assay commonly known as the Bradford assay 1 is widely used because of its rapid and convenient protocol as well as its relative sensitivityUnfortunately there is a large degree of curvature over a broad range of protein concentrations Fig.
Plotting the maximum absorbance values for each wavelength of light tested produces the samples absorbance spectrum and identifies the compounds making up the test substance and their proportions. Typical background absorbance after 2 hours incubation is 01 - 02 absorbance units. A Serial dilution is a series of dilutions with the dilution factor staying the same for each stepThe concentration factor is the initial volume divided by the final solution volume.
Instrument called a spectrophotometer and this absorbance is proportional to the concentration of the species being analyzed. A short summary of this paper. The absorbance value is plotted on the vertical y-axis against the wavelength of light used for a given test plotted on the horizontal x-axis.
Figure 4 is Beers Law curve for the absorbance of an iron-salicylate complex the substance prepared in todays experiment plotted against different concentrations. The concentration of NADH and NADPH solutions was checked by absorbance at 340 nm using a Shimadzu UV-120-02 spectrophotometer. Percent is as follows.
The pH value of an aqueous solution of 20 mM sodium phosphate is roughly 50 so in order to attain a pH value of 25 pH adjustment with some acid is required Depending on. The relationship between absorbance A and concentration c is known as the Beer-Lambert law. Percent molecular weight of protein Still other sources provide protein absorbance values for 01 mgmL solutions as this unit of measure is more.
Linearization of the Bradford Protein Calibration Graph. Spectroscopic measurements interpret the interaction between matter and light. Absorbance is defined by the equation A -logT or A logloI.
For example if you take 1 part of a sample and add 9 parts of water solvent then you have made a 110 dilution. Absorbance is defined as follows. J Plot a graph of time against concentration of MnO 4- ions.
Use sentences that draw the readers attention to the relationship or trend you wish to highlight referring to the appropriate Figure or Table only parenthetically. Derivative spectra If a spectrum is expressed as absorbance A as a function of wavelength λ the derivative spectra are. You can use Excel Trendlines to fit Polynomial relationships.
0 Aλ2 basic Aλ1 basic Aλ2 max pH Absorbance Aλ1 acidic I Aλ2 max 2 pKa Aλ2 acidic II FIGURE 2. Beers law describes the dependence of a proteins absorbance on its absorptivity coefficient its concentration and the pathlength of the incident light. This has a.
24 Full PDFs related to this paper. Molar 10 ε. A plot of absorbance as a function of pH for an indicator.

Assay For Enzyme Activity By Following The Absorbance Change Of Ph Indicators Sciencedirect

Pdf Spectrophotometric Ph Measurements In The Presence Of Dissolved Organic Matter And Hydrogen Sulfide Perturbations Of Spec Ph Measurements

Pdf Spectrophotometric Determination Of Ph Change Of Formation Water Under High Co2 Pressure Using A Mixed Ph Indicator

Relation Between Ph Values To Absorbance Download Scientific Diagram

Pdf Spectrophotometric Ph Measurements In The Presence Of Dissolved Organic Matter And Hydrogen Sulfide Perturbations Of Spec Ph Measurements

Pdf Spectrophotometric Determination Of The Pka Isosbestic Point And Equation Of Absorbance Vs Ph For A Universal Ph Indicator Semantic Scholar

Pdf Spectrophotometric Ph Measurements In The Presence Of Dissolved Organic Matter And Hydrogen Sulfide Perturbations Of Spec Ph Measurements

A Ph Determination Method Suitable For High Throughput Approaches Based On Spectral Absorbance

